A milestone achievement in botanical therapeutics
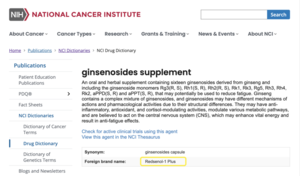
RICHMOND, BC, March 13, 2025 /24-7PressRelease/ -- Canada Royal Enoch Phytomedicine Ltd., an innovative leader in ginseng and ginsenoside research and application, proudly announces that its ginsenoside supplement, Redsenol-1 Plus Noble Ginsenosides, has been officially included in the National Cancer Institute's (NCI) Drug Dictionary. Redsenol-1 Plus is the only product in the global multi-component ginsenoside industry to receive this listing. This milestone underscores Canada Royal Enoch Phytomedicine's commitment to advancing cancer research and highlights the potential of Redsenol-1 Plus in oncology and supportive care.
The NCI Drug Dictionary serves as a premier reference for technical definitions and synonyms of drugs and agents used to treat cancer and related conditions. Redsenol-1 Plus is now defined within this authoritative database as "an oral and herbal supplement containing sixteen ginsenosides derived from ginseng, including the ginsenoside monomers Rg3(R, S), Rh1(S, R), Rh2(R, S), Rk1, Rk3, Rg5, Rh3, Rh4, Rk2, aPPD(S, R), and aPPT(S, R)..." This definition highlights a unique feature of Redsenol-1 Plus: its diverse profile of rare ginsenosides.
Unlike most ginseng extract supplements that primarily contain large-molecule, poorly absorbed prototype ginsenosides such as Rg1, Rb1, Rd, Re, and Rc, Redsenol-1 Plus features sixteen highly bioactive and absorbable rare ginsenosides at a concentration of 20%. This significantly surpasses conventional ginseng extract supplements, which typically contain only 4% total ginsenosides.
Canada Royal Enoch Phytomedicine has developed proprietary rare ginsenoside extraction, processing, transformation, and formulation technologies, allowing for the large-scale production of these valuable compounds. The company's technological advantage has positioned it as a global leader in ginsenoside innovation, setting it apart in the market.
"Redsenol-1 Plus's inclusion in the NCI Drug Dictionary offers researchers a standardized definition of its components," stated Dr. Peihua Yu, founder and CEO of Canada Royal Enoch Phytomedicine. "We remain dedicated to advancing the scientific understanding of ginsenoside applications in cancer-related conditions through rigorous research."
The inclusion of Redsenol-1 Plus in the NCI Drug Dictionary coincides with Canada Royal Enoch Phytomedicine's ongoing clinical trial on cancer-related fatigue (ClinicalTrials.gov Identifier: NCT05664009). The rigorous, triple-blind, placebo-controlled clinical study is being conducted by KGK Science, Inc., a leading Canadian clinical research organization. The trial is designed to evaluate the efficacy of Redsenol-1 Plus in mitigating cancer-related fatigue, a common and debilitating symptom experienced by cancer patients undergoing treatment. The success of this trial could position Redsenol-1 Plus as a groundbreaking solution for cancer-related fatigue.
About Canada Royal Enoch Phytomedicine Ltd.
Canada Royal Enoch Phytomedicine Ltd. is a biotechnology company specializing in the research, production, and sales of natural plant-based health products. The company's flagship brand, Redsenol® (also marketed as Redsenol-1, Redsenol-1 Plus, and Redsenol DAG), is a premier ginsenoside supplement brand featuring multicomponent rare ginsenosides in high concentrations. Through its proprietary technologies in rare ginsenoside extraction, processing, transformation, and formulation, Canada Royal Enoch Phytomedicine is committed to unlocking the full potential of ginseng and ginsenosides for health and wellness.
For more information on Canada Royal Enoch Phytomedicine, Redsenol-1 Plus and rare ginsenosides, visit https://enophyto.com.
---
Press release service and press release distribution provided by https://www.24-7pressrelease.com