ALBUQUERQUE, NM / ACCESSWIRE / October 30, 2024 / The Institute of Molecular Pathology and Immunology of the University of Porto (IPATIMUP), among the leading institutes for clinical digital pathology and research in the European Union, has partnered with Indica Labs to implement digital primary diagnostics with integrated AI-powered image analysis. IPATIMUP selected the HALO AP® enterprise digital pathology platform from Indica Labs for seamless integration of both off-the-shelf and third-party AI.
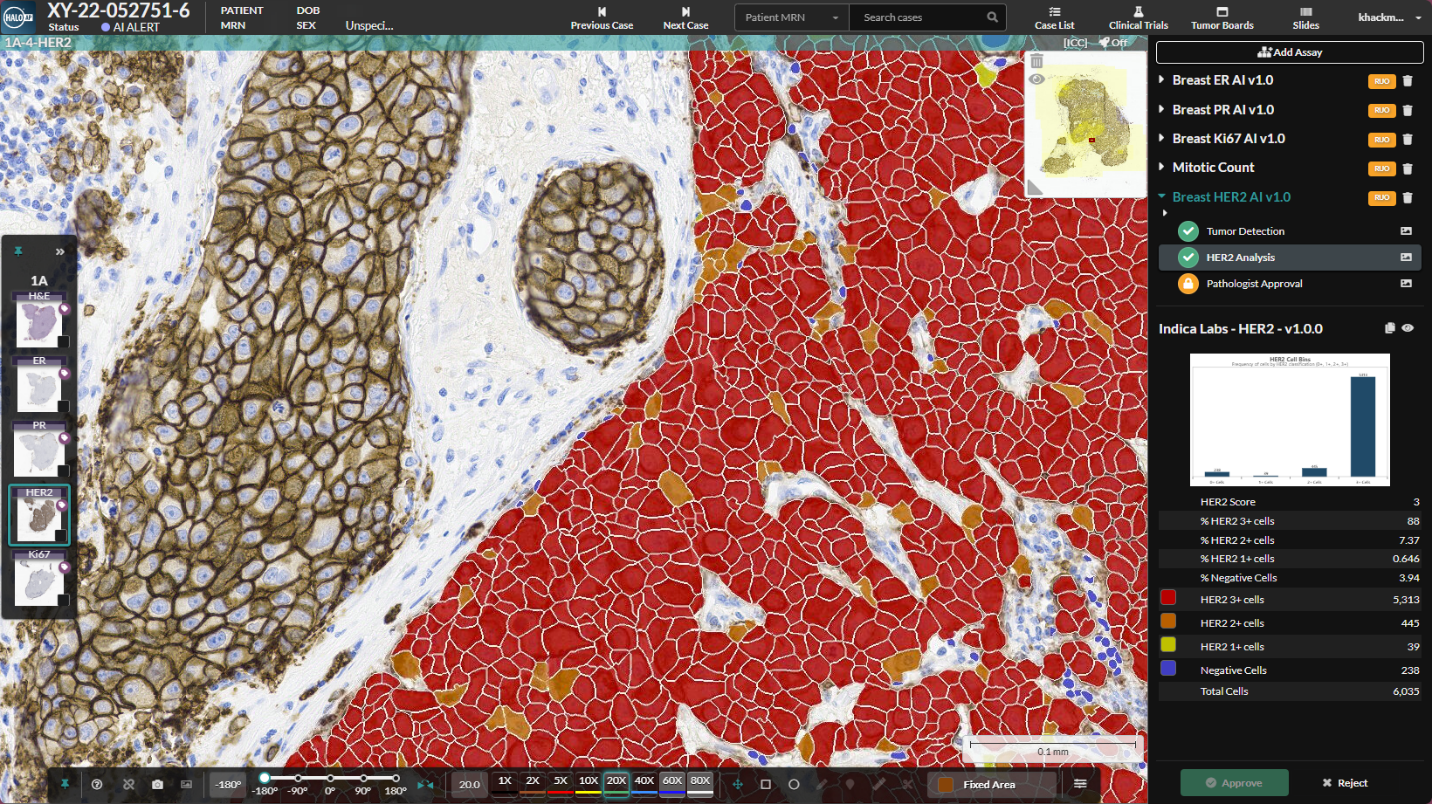
Breast IHC AI will be deployed in HALO AP® at IPATIMUP to power pathology workflows.
HALO AP® acts as an AI orchestration platform for the modern pathologist. IPATIMUP chose HALO AP® for their EU and CAP accredited laboratory because of its robustness, flexibility, and ability to accelerate diagnoses. The Institute will deploy Breast IHC AI and Lung PD-L1 AI from Indica Labs as in-house validated tests for automated quantification of IHC expression in cases of breast cancer and non-small cell lung cancer. In addition to these AI applications, the Institute plans to utilize Indica's HALO AI software for life science research to develop and validate their own AI algorithms for deployment in HALO AP® through a similar pathway.
Indica Labs offers a fully integrated pathology solution, creating a workflow that combines AI-powered tools with digital pathology. HALO Clinical AI offers advanced tools for cancer identification, grading, automated slide quality control, IHC expression quantification, and more. Beyond supporting AI-driven workflows, HALO AP® features tools for case management, remote viewing, and seamless integration with other laboratory systems, enhancing diagnostic precision, scalability, and accessibility.
"The deployment of HALO AP at IPATIMUP will allow us to dive into the important step of precise quantification of biomarkers," said Dr. Prof. Eloy, Head of the Pathology Laboratory of IPATIMUP and Professor of Medicine at The University of Porto. "The use of Breast IHC AI and Lung PD-L1 AI from Indica Labs, under the umbrella of our demanding quality control system, promises efficient and high-quality tools for the best patient care."
Leading institutions are adopting HALO AP® to streamline diagnostic workflows and drive downstream research pipelines. For organizations like IPATIMUP that operate at the intersection of clinical diagnostics and pathology research, the seamless and secure transition of data between these environments is critical. HALO AP® integrates with existing LIS | LIMS systems, ensuring data flows effortlessly. In addition, case and slide data can be easily interrogated within the platform and exported for research pipelines in a deidentified manner. By consolidating workflows under HALO AP®, pathologists can focus on delivering the best patient care.
"Artificial intelligence in pathology isn't about replacing human judgment - it's about amplifying it," said Peter Caie, Senior Principal Scientist in the AI Diagnostics group at Indica Labs. "By harnessing the power of AI, we're enabling pathologists to overcome technical barriers, improve turnaround times, and extract more meaningful data from each slide with reliable, consistent, and clinically validated support tools. We're thrilled to be able to assist IPATIMUP, a globally recognized leader in the adoption of digital pathology, in its critical work."
Breast IHC AI is For Research Use Only and not intended for clinical diagnostic use. Breast IHC AI is accessed via the HALO AP® enterprise digital pathology platform.
Lung PD-L1 AI is For Research Use Only and not intended for clinical diagnostic use. Lung PD-L1 AI is accessed via the HALO AP® enterprise digital pathology platform.
HALO AP® is CE-IVDR marked for in-vitro diagnostic use in Europe, the UK, and Switzerland. HALO AP® is For Research Use Only in the USA and is not FDA cleared for clinical diagnostic use. In addition, HALO AP® provides built-in compliance with FDA 21 CFR Part 11, HIPAA, and GDPR.
About Indica Labs
Indica Labs is the global leader in AI-powered digital pathology software and services. Our flagship HALO® and HALO AI platform revolutionizes quantitative evaluation of whole slide images. HALO Link provides collaborative image management while HALO AP® and HALO AP Dx deliver enterprise digital pathology for primary diagnosis with regulatory clearances in multiple markets. Through a commitment to open pathology, performance, scalability, and ease-of-use, we help pharma companies, diagnostic labs, hospitals, research organizations, and Indica's own Cloud and Pharma Services make discoveries and diagnoses that transform patient care and scientific discovery.
Indica Labs Media Contact:
Eric Runde
erunde@indicalab.com
SOURCE: Indica Labs
View the original press release on accesswire.com






